NATIONAL INSTITUTE OF SIDDHA
INTERMEDIARY PHARMACOVIGILANCE CENTRE
AYUSH OUSHADHI GUNVATTA EVAM UTTPADAN SAMVARDHAN YOJANA (AOGUSY)
Background
This component aims at developing safety monitoring mechanism for Ayurveda, Siddha, Unani and Homoeopathy (ASU&H) drugs and surveillance system to check the veracity of misleading advertisements of AYUSH. The proposed scheme of Pharmacovigilance has been envisaged towards detection, assessment, understanding, prevention and regulatory action of adverse events and misleading advertisement of ASU&H drugs.
Aim
- Inculcating the reporting culture among the consumers as well as ASU&H practitioners to facilitate documentation of Adverse Drug Reactions (ADRs) and instances of misleading advertisements of Ayurveda, Siddha, Unani & Homoeopathy drugs
- Developing a system-wise database of Adverse Drug Reactions of ASU & H drugs
- Evolving evidence based recommendations regarding the clinical safety and improper advertisements of ASU&H drugs for regulatory actions.
Structural Framework
A three tier structure comprising of a National Pharmacovigilance Co-ordination Centre (NPvCC), Intermediary Pharmacovigilance Centres (IPvCs) and Peripheral Pharmacovigilance Centres (PPvCs). The All India Institute of Ayurveda (AIIA), New Delhi, is the National Pharmacovigilance Co-ordination Centre (NPvCC) for the implementation of the National Pharmacovigilance program for ASU&H drugs. National Institute of Siddha is one of the Intermediary Pharmacovigilance Centres (IPvCs). There are fifteen Peripheral Pharmacovigilance Centres (PPvCs) established to take up the responsibility of safety monitoring of ASU&H drugs and reporting of their ADRs to National Institute of Siddha (NIS), IPvC.
Operational Guidelines
The National Pharmacovigilance Co-ordination Centre (NPvCC) as well as the Intermediary Pharmacovigilance Centres (IPvCs) also undertakes documentation and reporting of the ADRs.
An Intermediary Pharmacovigilance Centre (IPvC) has 10-20 Peripheral Pharmacovigilance Centres (PPvCs) under its purview. The Intermediary Pharmacovigilance Centre (IPvC) receive the information through the Peripheral Pharmacovigilance Centre (PPvC) as well as directly from the consumers, practitioners, industry and other stakeholders regarding any suspected Adverse Drug Reactions in the prescribed format and regarding misleading advertisements of Ayurveda, Siddha, Unani and Homoeopathic drugs on regular periodicity. Each Intermediary Pharmacovigilance Centre (IPvC) shall co-ordinate and receive inputs from Peripheral Pharmacovigilance Centres (PPvCs) selected from amongst recognized educational institutions; licensed ASU&H pharmacies; or accredited / Government approved ASU&H healthcare facilities, hospitals, practitioners and other stakeholders.
Responsibilities of the IPvC & PPvCs
The IPvCs of the respective systems (Ayurveda/ Siddha/ Unani/ Homoeopathy) shall-
- Document and monitor the ADRs of ASU&H drugs
- Collect the information regarding the ADRs of ASU&H drugs from the respective Peripheral Pharmacovigilance Centres (PPvCs)
- Report the ADRs of ASU&H drugs to the National Pharmacovigilance Centre (NPvCC) at regular intervals for casualty assessment
- Organize awareness building and capacity building workshops for the stakeholders
- Scrutinize the project proposals received from the Peripheral Pharmacovigilance Centres (PPvCs) and forward the same to the National Pharmacovigilance Centre (NPvCC) along with comments.
The PPvCs of the respective systems (Ayurveda/ Siddha/Unani/Homoeopathy) shall-
- Document and monitor the ADRs of ASU&H drugs
- Report the ADRs of ASU&H drugs to the concerned Intermediary Pharmacovigilance Centre (IPvC) at regular intervals
- Report misleading advertisements to respective State Licensing Authority with information to the Intermediary Pharmacovigilance Centre. Action taken report of the same need to be submitted on monthly basis
- Conduct awareness programs under the guidance of IPvC and NPvCC
Till 2023 October, IPvC has reported 63 ADRs totally. Awareness Programmes have been conducted for PG Scholars, All Hospital Staffs, OPD Patients periodically. Misleading Advertisements also have been reported to State Licensing Authority (SLA) every month.
Details of IPvC Coordinators:
Dr. P. Shanmugapriya MD(S)., Ph.D.,
Professor, Department of Nanju Maruthuvam, IPvC, NIS.
Dr. S. Sudha Revathy MD(S)., Ph.D.,
Assistant Professor, Department of Gunapadam, IPvC, NIS.
Research Associate:
Dr. P. Kamalasoundaram MD(S)., IPvC, NIS.
Data Entry Operator:
Ms. H.S. Zareena IPvC, NIS.
Adverse Drug Reaction (ADR) reporting:
Patient / Attendant / Nurse / Doctor / Pharmacist / Health worker / Drug Manufacturer / anyone can report ADR to NIS, IPvC or nearby any PPvCs.
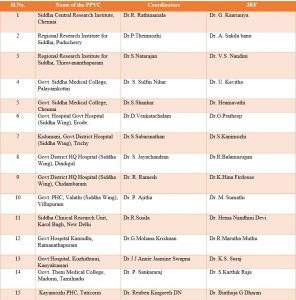
Details of Peripheral Pharmacovigilance Centres (PPvCs)
Click here to download ADR form ![]()
Click here to download AOGUSY Scheme Guideline ![]()